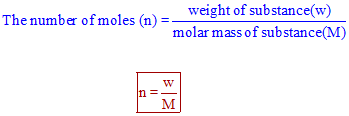Using the information in the table, calculate the number of moles in a 7.89 kg sample of - Brainly.com

How to Find Moles of Product from Moles of Reactant using a Chemical Equation | Chemistry | Study.com
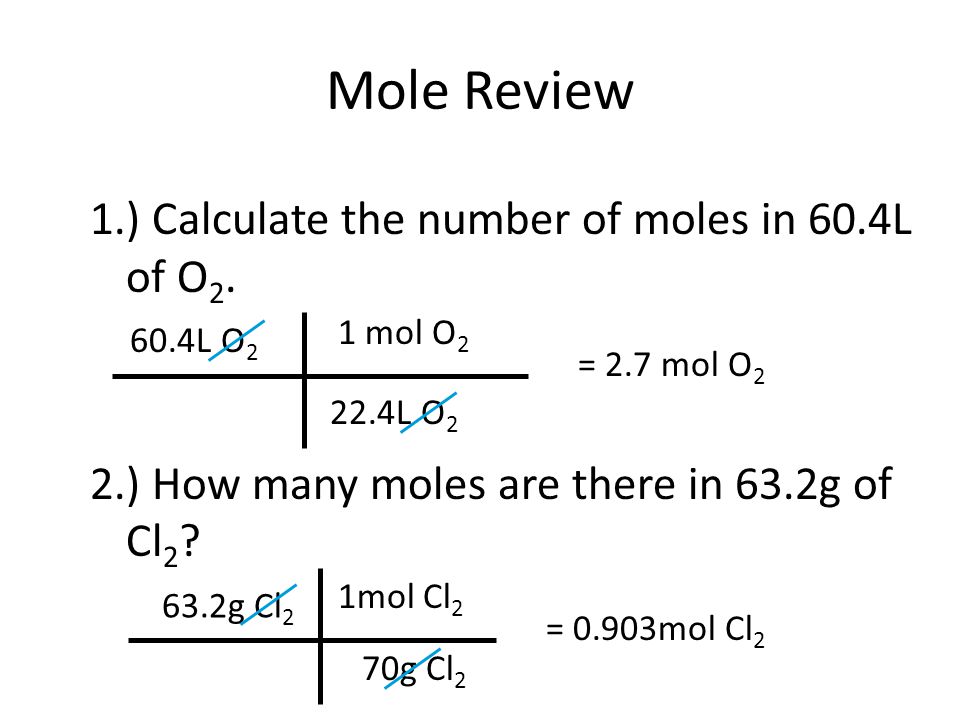
Mole Review 1.) Calculate the number of moles in 60.4L of O2. 2.) How many moles are there in 63.2g of Cl2? 1 mol O2 60.4L O2 = 2.7 mol O2 22.4L
calculate the no of moles present in 1 litre of water if the dw c alculate the no of moles present in 1 l of water if the density of water is

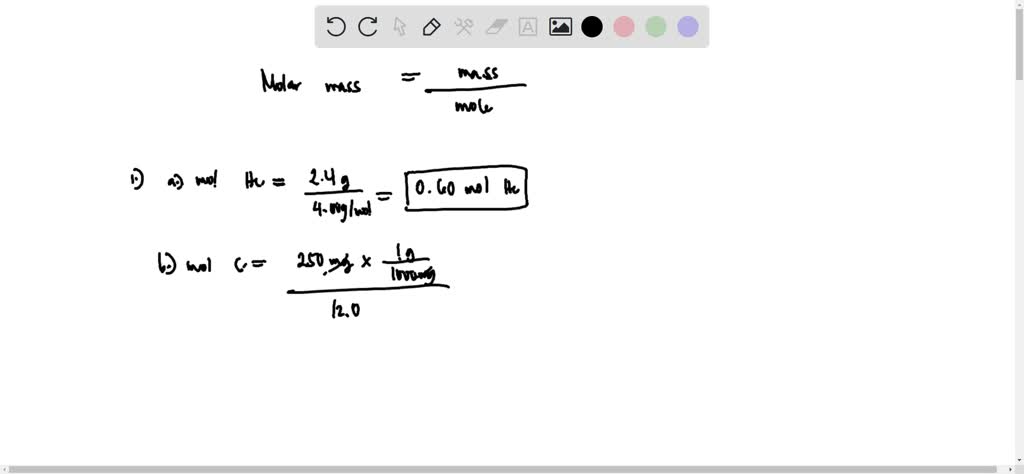
SOLVED: Calculate the number of moles in each sample. a. 72.5 g CCl4 b. 12.4 g C12H22O11 c. 25.2 kg C2H2 d. 12.3 g of dinitrogen monoxide

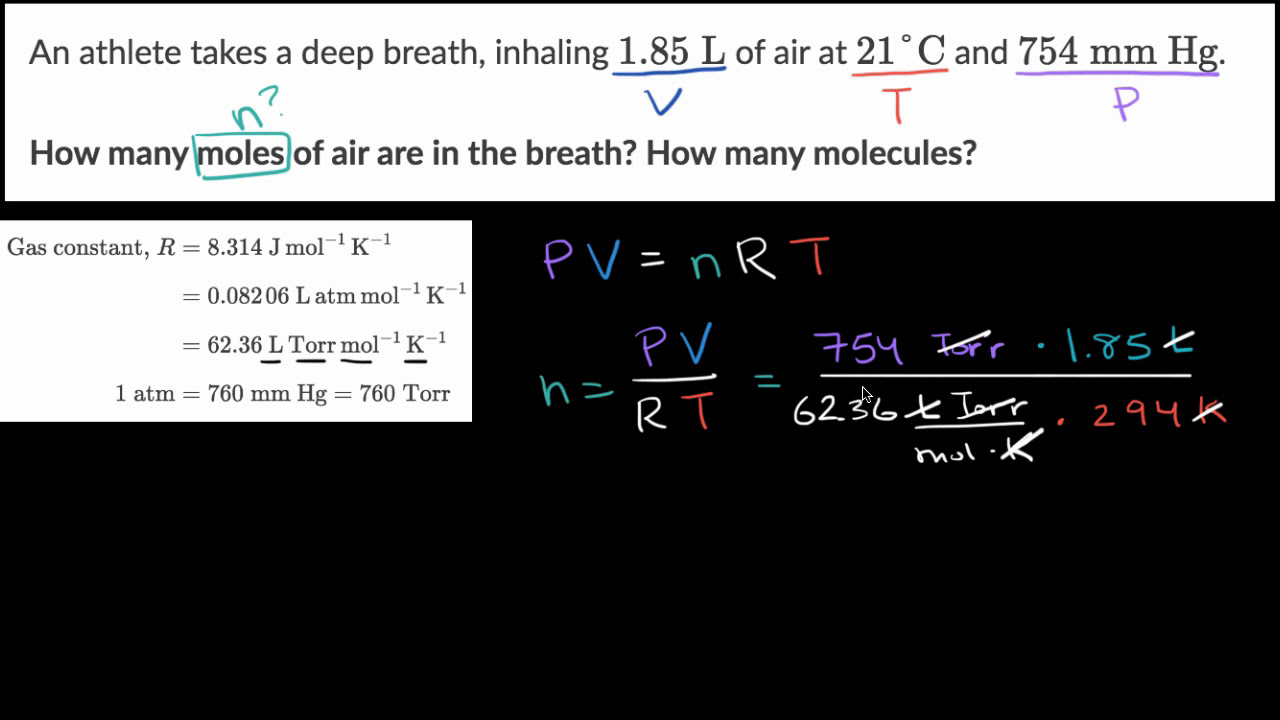
Calculate the number of moles for the following: (i) 52 g of He (finding mole from mass) (ii) `1... - YouTube

Calculate the number of moles for the following: 52 g of He (finding mole from mass) 12.044 × 10^ 23 number of He atoms (finding mole from number of particles)

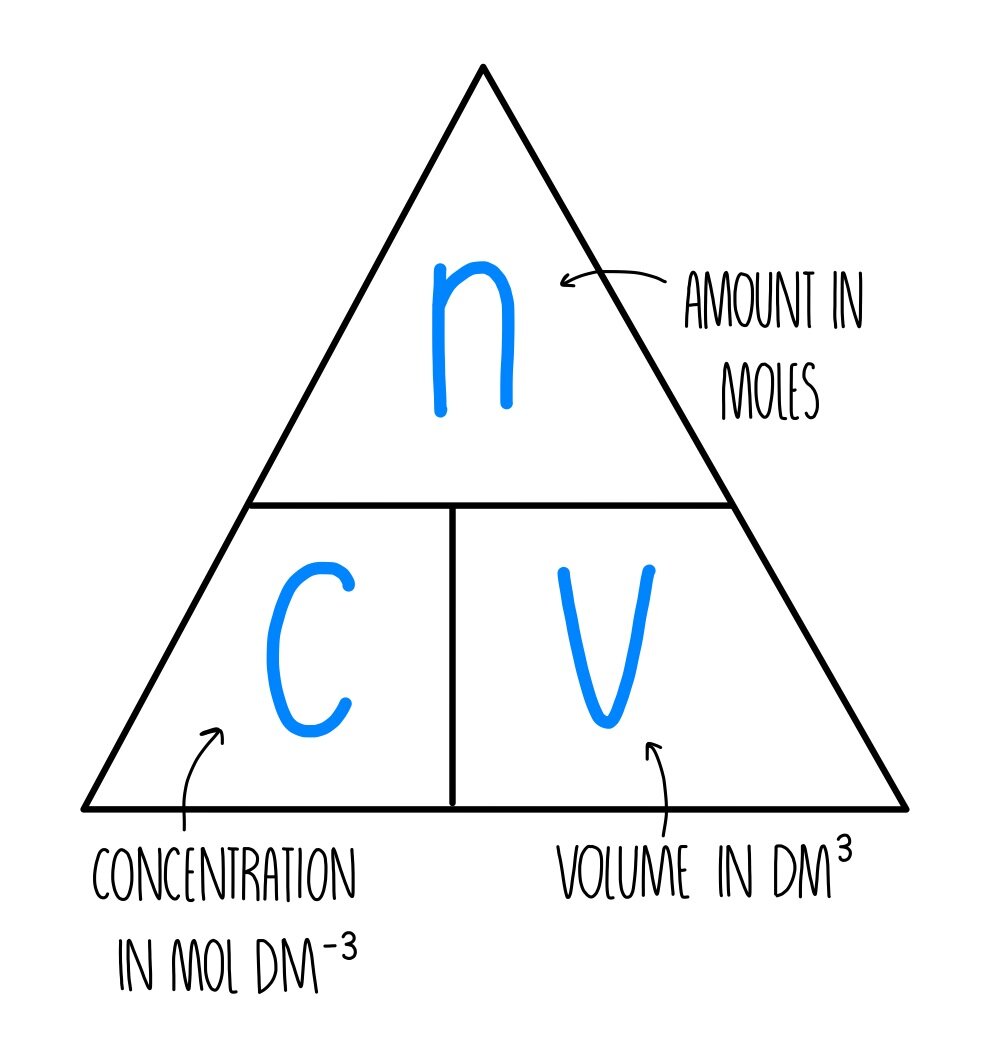
Moles and Solutions g n gfm To calculate the number of moles in a solution we use the following n CV n = number of moles C = concentatration (mol/l) V. - ppt download